How To: Determine on Which TMF Level a Document Should Be Filed
As discussed in our recent newsletter entry, How To: Use the TMF Reference Model, the TMF Reference Model is an industry-adopted index that has increased TMF quality and ease of use. We used columns A through H of the TMF Reference Model to correctly file a document by determining its Zone, Section, and Artifact. Now let’s turn our attention to columns T through Y of the TMF Reference Model, TMF Level, introducing a new dimension of document indexing in the TMF by Trial, Country, and Site Level.
A document’s TMF Level is a type of metadata. Metadata is information attached to a document’s file that isn’t necessarily in the document itself (for example, filing date, version number, author, or title). A document’s Level represents its place in the hierarchy of clinical trial conduct. This hierarchy (Trial, Country, and Site Level) reflects how regulatory responsibility is delegated within a trial and mirrors the natural divisions between the trial events and documents of the trial site, the regional or country offices of the Sponsor, and the Sponsor’s global headquarters.

Screenshot of the TMF Reference Model Home Page with link to the current version (Version 3.2.1) of the TMF Reference Model.
In the past, when trials were conducted with paper TMFs, the TMF would, by necessity, be divided into three document repositories (site, regional office, and sponsor’s headquarters). These three document repositories would only become physically combined at the closure of a trial. Now with modern eTMF systems, documents can be accessed by anyone at any time. Although documents are now freed from these physical limitations, modern eTMFs and the TMF Reference Model still preserve the distinction between these three repositories of documents (which are now called Levels). Regulators want to know a document’s Level because it conveys important context about to whom a document applies, for what purposes it was created, and from where the document came.
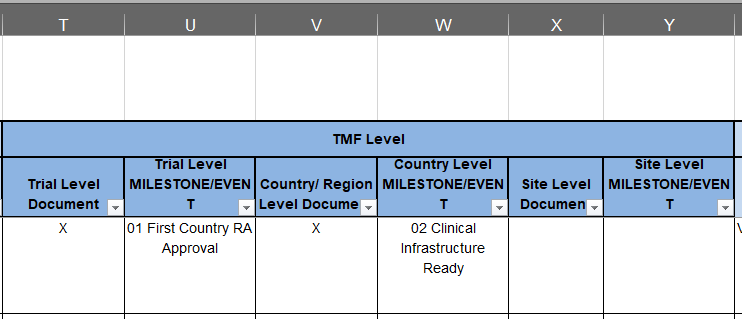
Screenshot of TMF Level columns of the TMF Reference Model.
If you still have your copy of the TMF Reference Model available from our last newsletter entry, take a look at columns T, V, and W. You’ll see these three columns that are titled ‘Trial Level Document’, ‘Country/Region Level Document’, and ‘Site Level Document’. For each artifact row in the spreadsheet, you will see that at least one of the three TMF Level columns are marked with an X. Looking at the key in row 1 at the top of the spreadsheet, you’ll see that an X means applicable. An X in each column indicates a certain type of document could be filed on that TMF Level. A document’s TMF Level gives the following information:
Trial Level: Documents filed in the Trial Level impact the widest number of stakeholders. Trial Level documents tell the story of the key management decisions of a trial and demonstrate the sponsor’s oversight. They may also act as the basis or master template of documents that are customized at Country or Site Level. Documents that show the topline results of a trial (like the Clinical Study Report and tables, listings, and figures) will also be filed here once the trial has ended.
Country Level: Often containing the fewest number of documents when compared to the two other levels, documents filed in Country (or region) are related to country- or region-specific regulatory requirements. Commonly filed Country Level documents include ICF master templates customized for a country and regulatory body submissions and supporting documents, such as an MHRA approval or EudraCT registration document. If the trial occurs in one region or country, especially if the trial is small, the Country Level may be merged into the Trial Level.
Site Level: Documents filed here were produced by a trial site or documents customized for use at a specific trial site. Common examples include documents that are a part of the regulatory packet (CVs, 1572s, and medical licenses) as well as IRB approvals specific to an individual site. Trial and Country Level document templates customized for a specific site would also be filed in the Site Level.
While designed for advanced users of the TMF Reference Model, columns C, D, and E of the “Milestones_Events & Description” sheet are a useful resource to help you anticipate how certain study milestones and documents can simultaneously impact multiple TMF Levels. These milestones also correspond with the “Trial Level MILESTONE/EVENT” columns T, V, and W of the TMF Reference Model described above. For example, an initial regulatory authority approval (milestone #1, FIRST COUNTRY RA Approval) could trigger the creation of documentation that should be filed at all (Trial, Country, and Site) TMF Levels.
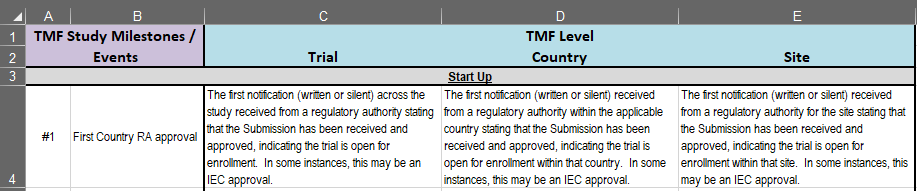
Screenshot of columns C, D, and E of the “Milestones_Events & Description” sheet of the TMF Reference Model
So, when struggling to determine a document’s TMF level, it’s best to seek more information from the subject matter experts who know the story of a clinical trial best.
The following guidance is given in the TMF Refence Model User Guide regarding TMF Levels: “It is important to note that artifacts should be filed at the most appropriate level, based on the content of the record.” The TMF Reference Model, the user guide reminds us, is not a one-size-fits-all solution. Just as study-specific circumstances can make determining the correct Zone, Section, or Artifact a challenge, so too can study-specific circumstances impact a document’s Level.
Sometimes, even after great consideration, you may find a document can be filed within multiple Levels. For this reason, it’s crucial that your TMF plan provides enough detail to ensure consistent filing practices across your whole team. More than just the identity of an essential document presented through its Zone, Section, and Artifact, TMF Level allows you to capture essential information about a document’s place within the hierarchy of site, country, and trial—information that is critical to anyone seeking to reconstruct the narrative of a clinical trial through your TMF.

